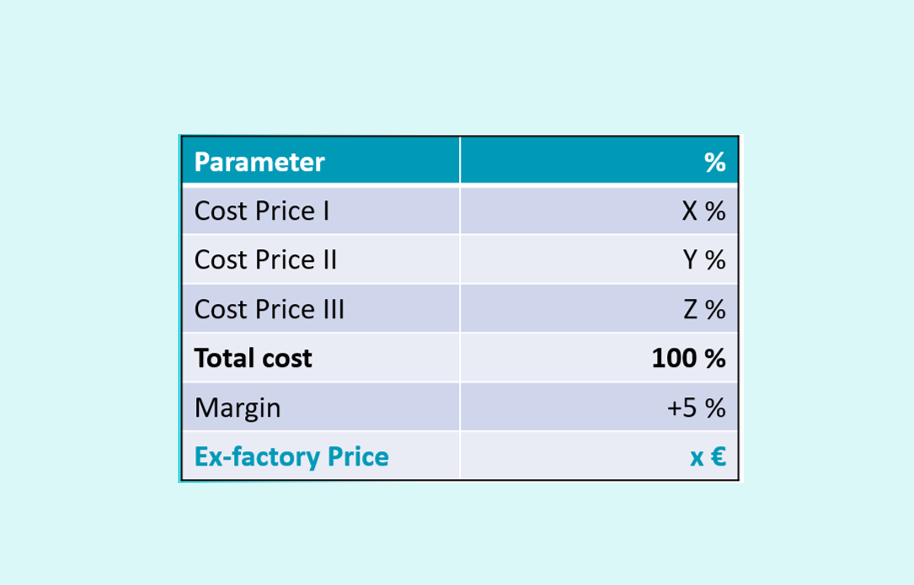
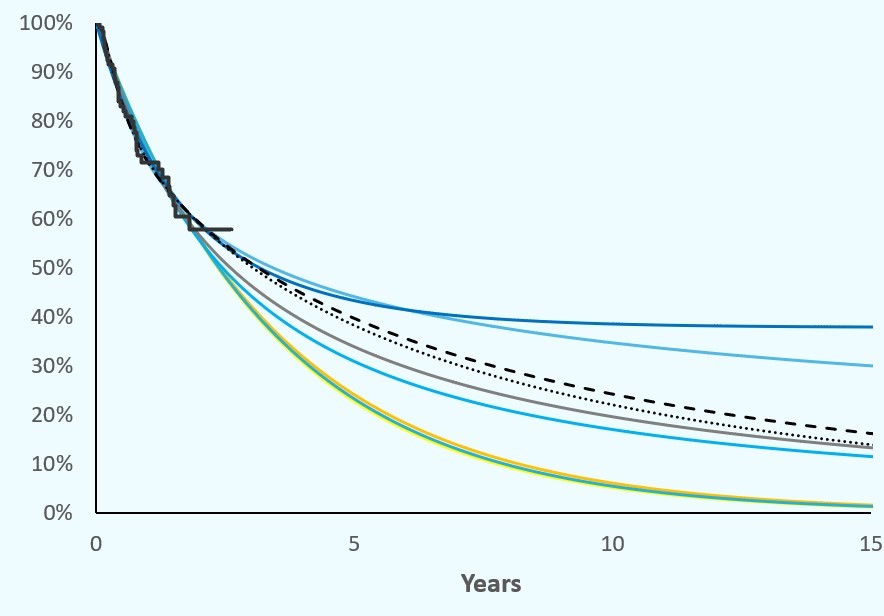
The value of a proven pricing structure
Added on 30/01/2024
How to prove the concerned price if the specialty is not yet produced? What is the value of a proven pricing structure if the price is downsized over time? Lets discuss to overcome this bottleneck.